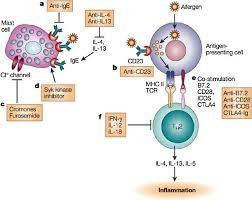
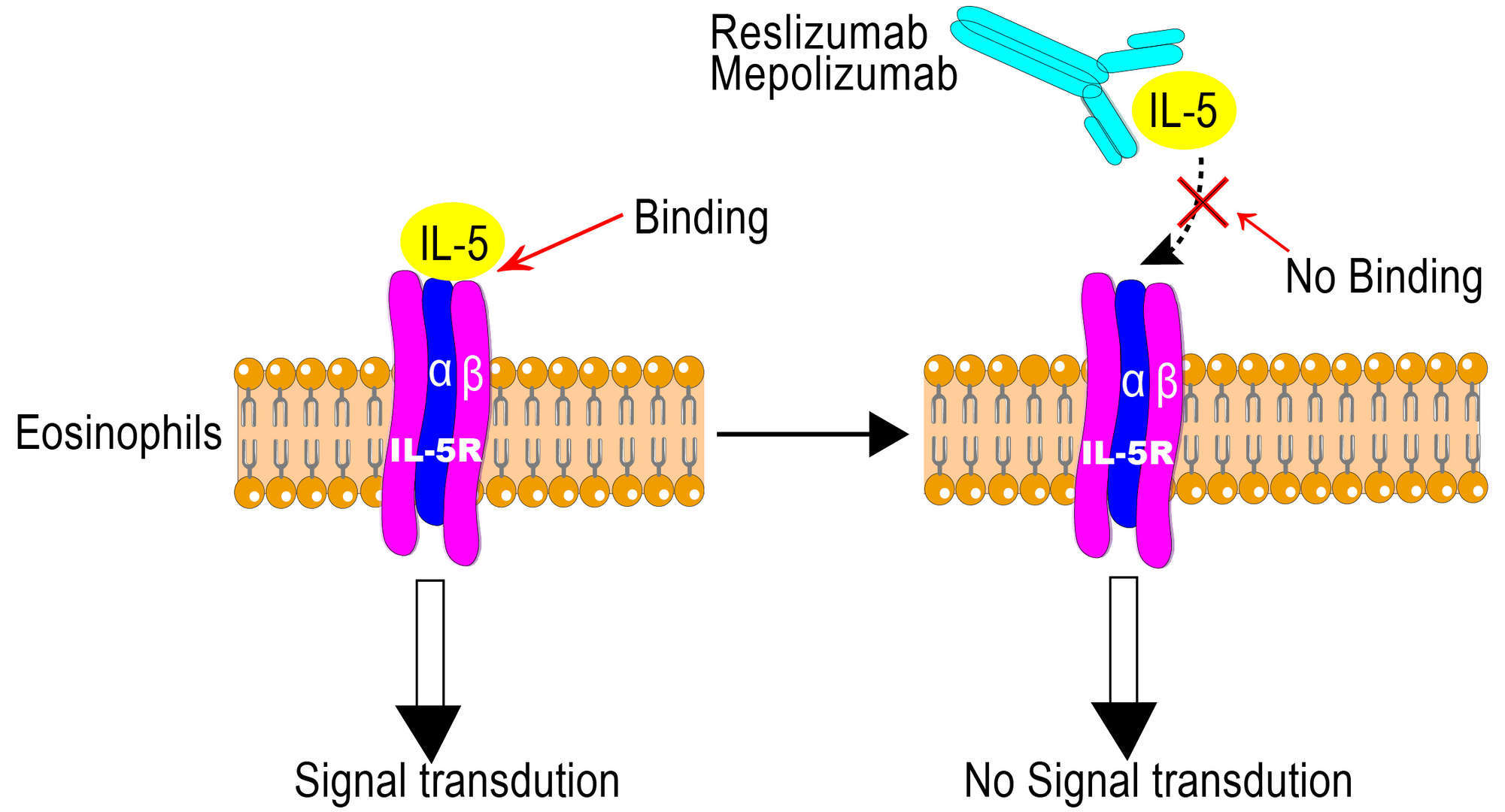
Reslizumab in the treatment of inadequately controlled asthma in adults and adolescents with elevated blood eosinophils: clinical trial evidence and future prospects - Jorge Máspero, 2017

Cinqair (reslizumab) Injection for treatment of Severe Asthma and an Eosinophilic Phenotype - Clinical Trials Arena
CINQAERO (reslizumab) de Teva ya está disponible en España para el asma grave no controlada | IM Farmacias
Clinical Study Protocol Distribution of Eosinophils in Asthma after Reslizumab (DEAR). A 7-week, Placebo- Controlled, Double-Bli

Real-World Effectiveness of Reslizumab in Patients With Severe Eosinophilic Asthma – First Initiators and Switchers - ScienceDirect
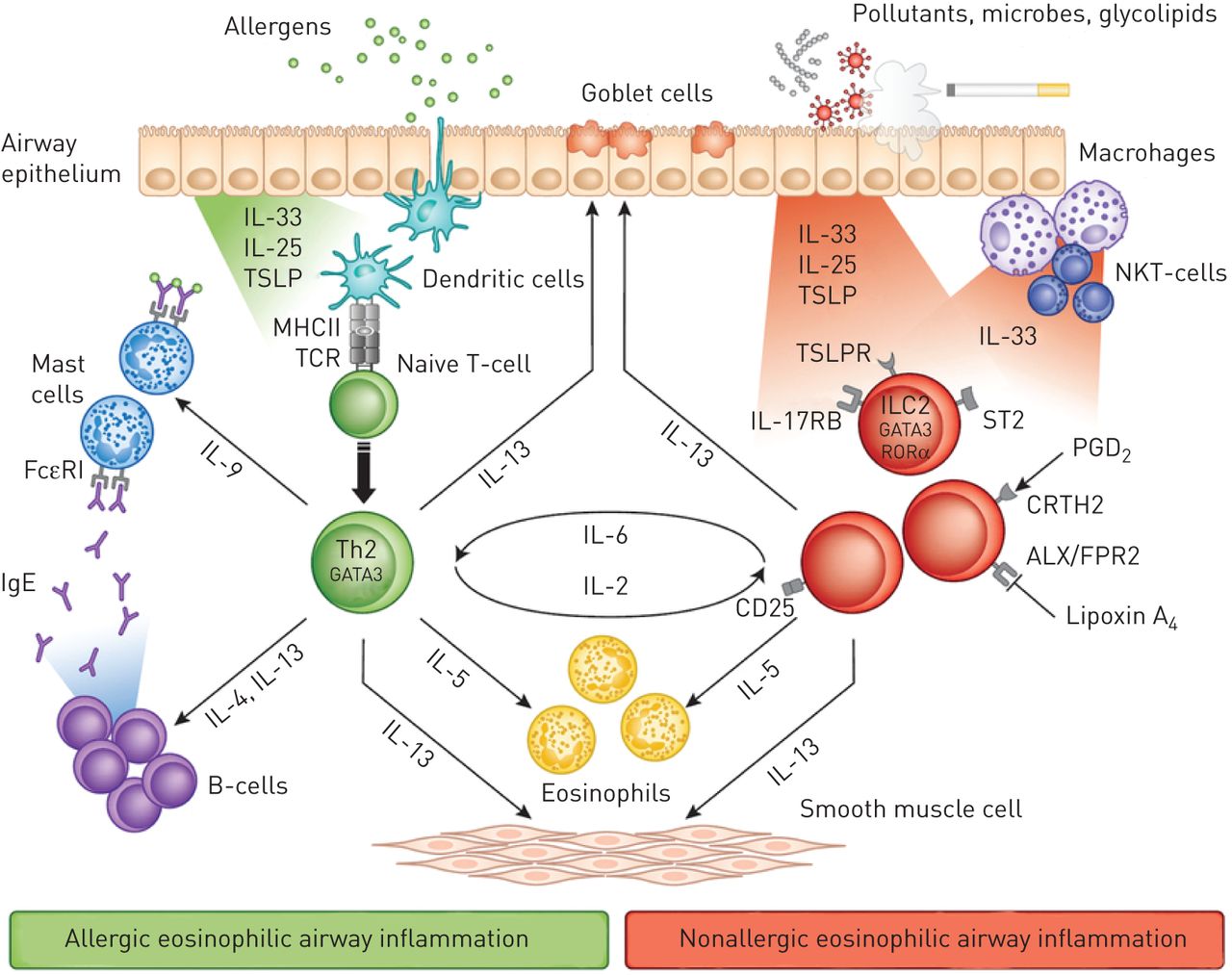
Israeli Pharma gets FDA Approval for New Antibody against Severe Asthma | Free directory of clinics in Israel